WaterWetter®
TECHNICAL
INFORMATION
Red
Line WaterWetter® is designed to provide improved metal wetting
and excellent corrosion inhibition when added to plain water or a glycol
coolant. The most poorly maintained system in an automobile is usually
the cooling system. Maintenance is quite simple and only required once
each year, but most vehicle owners never routinely change the coolant
or replenish the corrosion inhibitors which are required for trouble-free
operation. Proper cooling system maintenance is very critical for most
modern engines which utilize more aluminum. Aluminum has a very high
corrosion potential, even higher than zinc, which is very widely used
as a sacrificial anode. The only property which enables aluminum to
be used in a cooling system is that it will form protective films under
the proper conditions which will prevent the uncontrolled corrosive
attack of acids or bases. Poor aluminum corrosion inhibition will cause
the dissolution of aluminum at the heat rejection surfaces, weakening
the cooling system walls and water pump casing and weakening the head
gasket mating surfaces. These corrosion products will then form deposits
on the lower temperature surfaces such as in radiator tubes which have
very poor heat transfer properties, causing a significant reduction
in the cooling ability of the entire system. Red Line WaterWetter®
will provide the proper corrosion inhibition for all cooling system
metals, including aluminum, cast iron, steel, copper, brass, and lead.
Water
has twice the heat transfer capability when compared to 50% glycol antifreeze/coolant
in water. Most passenger automobiles have a cooling system designed
to reject sufficient heat under normal operating conditions using a
50/50 glycol solution in water. However, in racing applications, the
use of water and WaterWetter® will enable the use of smaller radiator
systems, which means less frontal drag, and it will also reduce cylinder
head temperatures, even when compared to water alone, which means more
spark advance may be used to improve engine torque.
BENEFIT SUMMARY
- Doubles the wetting
ability of water
- Improves heat
transfer
- Reduces cylinder
head temperatures
- May allow more
spark advance for increased torque
- Reduces rust,
corrosion and electrolysis of all metals
- Provides long
term corrosion protection
- Cleans and lubricates
water pump seals
- Prevents foaming
- Reduces cavitation
corrosion
- Complexes with
hard water to reduce scale
COOLING SYSTEM REQUIREMENTS
The
conventional spark ignition gasoline engine is not a very efficient
powerplant. A considerable amount of the available fuel energy must
be rejected from the metal combustion chamber parts by the coolant and
dispersed to the atmosphere through the radiator. This heat rejection
is necessary in order to prevent thermal fatigue of the pistons, cylinder
walls, and the cylinder head. Another problem is that the combustion
chamber must be cooled enough to prevent preignition and detonation.
The higher the combustion chamber temperatures, the higher the octane
number required to prevent preignition and detonation. Since the octane
of the available fuel is limited, increasing temperatures in the combustion
chamber require retarding the spark timing which reduces the peak torque
available. Higher inlet temperatures also reduce the density of the
fuel/air mixture, reducing available torque further. For these reasons
reducing the flow of heat to the coolant usually reduces the efficiency
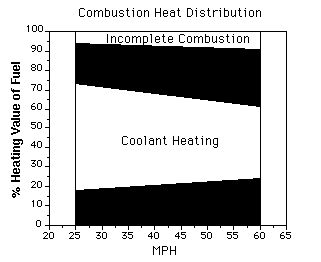
of the engine. Figure 1 shows a typical heat balance diagram for a spark
ignition engine. This diagram demonstrates that the coolant in an automobile
engine must absorb and reject through the radiator 2 to 3 times the
amount of energy which is converted to brake power.
THERMAL PROPERTIES
Water
has amazingly superior heat transfer properties compared to virtually
any other liquid cooling medium - far superior to glycol-based coolants.
As shown in Table 1, water has almost 2.5 times
greater thermal conductivity compared to glycol coolants. Mixtures of
glycol and water have nearly proportional improvement due to the addition
of water. Most heat is transferred in a cooling system by convection
from hot metal to a cooler liquid as in the engine block or from a hot
liquid to cooler metal surfaces, as in the radiator. The convection
coefficient of liquids in a tube is a complicated relationship between
the thermal conductivity, viscosity of the liquid, and the tube diameter
which determines the amount of turbulent flow. Since 50/50 glycol solution
has about 4 times the viscosity and only 70% of the thermal conductivity
of water, the thermal convection coefficient for a 50/50 glycol solution
is approximately 50% of the coefficient for water. Water in the cooling
system is capable of transferring twice as much heat out of the same
system as compared to a 50/50 glycol coolant and water solution. In
order for a 50/50 glycol mixture to reject as much heat as water (amount
of heat rejected is independent of the coolant), the temperature differentials
at the heat transfer surface must be twice as great, which means higher
cylinder head temperatures.
Table 1
Thermal Properties of Cooling System Materials
| Material |
Density
g/cm3 |
Thermal
Conductivity
Watt/m · °C |
Thermal
Convection
Watt/m · °C |
Heat
Capacity
cal/g · °C |
Heat of
Vaporization
cal/g |
| Water |
1.000 |
0.60 |
1829 |
1.000 |
539 |
| Glycol |
1.114 |
0.25 |
------ |
0.573 |
226 |
| 50/50 |
1.059 |
0.41 |
897 |
0.836 |
374 |
|
| Aluminum |
2.70 |
155 |
|
0.225 |
|
| Cast Iron |
7.25 |
58 |
|
0.119 |
|
| Copper |
8.93 |
384 |
|
0.093 |
|
| Brass |
8.40 |
113 |
|
0.091 |
|
| Ceramics |
|
1 - 10 |
|
|
|
| Air |
.0013 |
.026 |
|
0.240 |
|
HEAT TRANSFER
Red
Line WaterWetter® can reduce cooling system temperatures compared
to glycol solutions and even plain water. Water has excellent heat transfer
properties in its liquid state, but very high surface tension makes
it difficult to release water vapor from the metal surface. Under heavy
load conditions, much of the heat in the cylinder head is transferred
by localized boiling at hot spots, even though the bulk of the cooling
solution is below the boiling point. Red Line's unique WaterWetter®
reduces the surface tension of water by a factor of two, which means
that much smaller vapor bubbles will be formed. Vapor bubbles on the
metal surface create an insulating layer which impedes heat transfer.
Releasing these vapor bubbles from the metal surface can improve the
heat transfer properties in this localized boiling region by as much
as 15% as shown in Figure 2. This figure demonstrates the removal of
heat from an aluminum bar at 304°F by quenching the bar in different
coolants at 214°F under 15 psi pressure. Compare the time required
to reduce the temperature of the aluminum to 250°F, or the boiling
point of water at 15 psi. WaterWetter® required 3.2 seconds, water
alone 3.7 sec, 50/50 glycol in water required 10.2 sec, and 100% glycol
required 21 sec. Water alone required 15% longer, 50/50 glycol 220%
longer, and 100% glycol required 550% longer.
 |
|
Performance
Properties of Coolants
| Cooling System Fluid |
Stabilized Temperature |
| 50% Glycol/ 50% Water |
228°F |
| 50/50 with WaterWetter |
220°F |
| Water |
220°F |
| Water with WaterWetter |
202°F |
|
DYNO TEST RESULTS
Dynomometer
tests performed by Malcolm Garrett Racing Engines showed significant
improvements in coolant temperatures using WaterWetter. These tests
were performed with a Chevrolet 350 V-8 with a cast iron block and aluminum
cylinder heads. The thermostat temperature was 160°F. The engine
operated at 7200 rpm for three hours and the stabilized cooling system
temperature was recorded and tabulated below:
These numbers are
similar to the temperatures recorded in track use and heavy-duty street
use.
COOLANT EFFECTS
ON PERFORMANCE
Under
moderate load conditions, each percent glycol raises cylinder head temperatures
by 1°F. 50% glycol raises head temperatures by 45°F. This
increase in temperature will raise the octane required for trace knock
levels by typically 3.5 octane numbers. A car equipped with a knock
sensor will retard the timing to compensate for the increase in octane
requirement by approximately 5°, which will reduce the maximum
brake torque by about 2.1%. Racing vehicles not equipped with knock
sensors can advance timing for increased torque.
BOILING POINT ELEVATION
Red Line
WaterWetter® does not significantly increase the boiling point of
water; however, increasing pressure will raise the boiling point. The
boiling point of water treated with Red Line using a 15 psi cap is 250°F
compared to 265°F at 15 psi for 50% glycol. Increasing the pressure
by 50% to 23 psi will increase the boiling point of water to 265°F.
Because of the doubling of the ability of the radiator to transfer heat,
boilover using Red Line treated water is not a problem as long as the
engine is circulating coolant through the head and the fan is circulating
air. Sudden shutdown after very hard driving may cause boilover.
| SAE 880266 |
Water +
Red Line |
50% Glycol |
70% Glycol |
Increase in Cylinder
Head Temperature |
Baseline |
+45°F |
+65°F |
Increase in Octane
(RON) Requirement |
Baseline |
+3.5 |
+5.0 |
Change in Spark Timing
for Trace Knock |
Baseline |
-5.2° |
-7.5° |
| Change in Torque |
Baseline |
-2.1% |
-3.1% |
FREEZING POINT DEPRESSION
Red
Line WaterWetter® does not significantly reduce the freezing point
of water. If the vehicle will see freezing temperatures, an antifreeze
must be used. Water expands approximately 9% upon freezing which can
cause severe engine damage. Even in summertime, the use of air-conditioning
can blow freezing air through the heater and cause freezing of the heater
core unless approximately 20% antifreeze is used.
CORROSION PROTECTION
Modern
automotive engines now use aluminum for heads, radiators, water pump
housings, and nearly all hose fittings. These engines require significantly
greater corrosion protection than their cast iron counterparts of the
past. Aluminum is such an electroactive metal that it requires an impenetrable
corrosion inhibitor film to prevent rapid corrosion. Acid neutralization
capability is very important. Coolant which has been left in a cooling
system for several years has probably become acidic from the oxidation
of the glycol to acids. Also, keeping the glycol concentration in the
cooling system below 50% will help stability.
Red
Line also provides excellent protection from cavitation erosion in the
water pump and cylinder head. Localized boiling in the cylinder head
forms vapor bubbles which collapse when they come in contact with cooler
liquids. This collapse creates tremendous shock waves which removes
the inhibitor film from the aluminum surface and can cause catastrophic
erosion of the aluminum if the inhibitor does not reform the film quickly.
Another problem created by cavitation erosion is the deposition of the
removed aluminum as a salt with poor heat transfer properties in the
lower temperature radiator tubes. Red Line prevents this corrosion through
effective film formation and smaller vapor bubble formation, which has
a less violent collapse. Foam control is equally important since entrained
air will cause cavitation erosion due to the collapse of foam bubbles.
Red Line provides excellent control of foam with water alone and glycol
solutions.
Most
coolants additives on the market provide only protection for iron and
perhaps moderate protection for aluminum. The milky soluble oil types
can actually impede heat transfer by wetting the metal surface with
oil and this oil can swell and soften rubber coolant hoses. Table 3
shows the many tests which the Red Line formula will satisfy and how
it compares to a standard antifreeze.
TABLE 3
Comparison of Corrosion Inhibition Properties |
| PROPERTY |
RED LINE |
SPEC |
COOLANT A |
| pH |
8.6 |
7.5 - 11 |
9.8 |
| Boiling Point @ 15 psig |
250°F |
|
265°F (50%) |
| Freezing Point |
31°F |
-35°F(50%) |
-35°F |
| Foaming Height, ml |
75 |
150 |
50 |
| Color |
Pink |
|
green |
| Ash, % |
0.5 |
5, max |
1 |
Surface Tension @ 100°C,
Dynes/cm2 |
28.3 |
58.9 (water) |
|
ASTM D4340 Heat Transfer
Corrosion Test, Aluminum
Weight loss, mg/cm2/wk |
0.21 |
1 max |
0.45 |
ASTM D1384 Corrosion,
Weight loss, mg/specimen |
|
|
|
| Copper |
1 |
10 max |
5 |
| Solder |
6 |
30 |
7 |
| Brass |
2 |
10 |
5 |
| Steel |
1 |
10 |
6 |
| Cast Iron |
0 |
10 |
3 |
| Aluminum |
16 |
30 |
30 |
SLIPPERINESS OF
COOLANTS
Red
Line WaterWetter® does not alter the frictional property of tire
rubber and water on a pavement surface. The chart below shows the static
and dynamic friction of pavement wetted with different coolant types.
Higher friction indicates less slipperiness. The dynamic friction indicates
the increase in slipping which occurs after the tire begins to break
loose. Water and water with WaterWetter® reduce the friction relative
to dry pavement about 50%, but it is much less than the reduction in
friction caused by ethylene glycol and even more slippery is propylene
glycol.
USE DIRECTIONS
One
12 ounce bottle treats 12-16 quarts of water or a 50% ethylene or propylene
glycol solution. In smaller cooling systems, use 4-5 caps per quart.
Add directly through the cooling system fill cap into the radiator or
into the overflow tank. Do not open a cooling system while hot. For
best protection for aluminum, replenish or replace every 15,000 miles.
The anti-scaling ingredients in Red Line WaterWetter allow its use with
ordinary tap water. However, using with distilled or deionized water
will accomplish some scale removal in the cylinder head area. For maximum
temperature reductions use the most water and the least antifreeze possible
to prevent freezing in your climate. Even in summertime the use of air-conditioning
can blow freezing air through the heater and cause freezing of the heater
core unless approximately 20% antifreeze is used. Red Line WaterWetter
is available in 12 ounce containers.
Red Line Synthetic
Oil Corp.
6100 Egret Court
Benicia, CA 94510
(707)745-6100
© 2000
Red Line Synthetic Oil Corp.
|